EMIG GmbH - Official distributor of medical equipment in the following regions of Europe and beyond:
- Meyer & Haake – Ukraine, Austria, Germany (Bavaria); RiwoSpine – Ukraine, Moldova, Estonia;
- Inomed – Ukraine, Moldova, Estonia; Fluoptics – Ukraine.
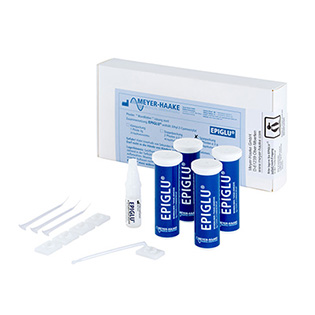
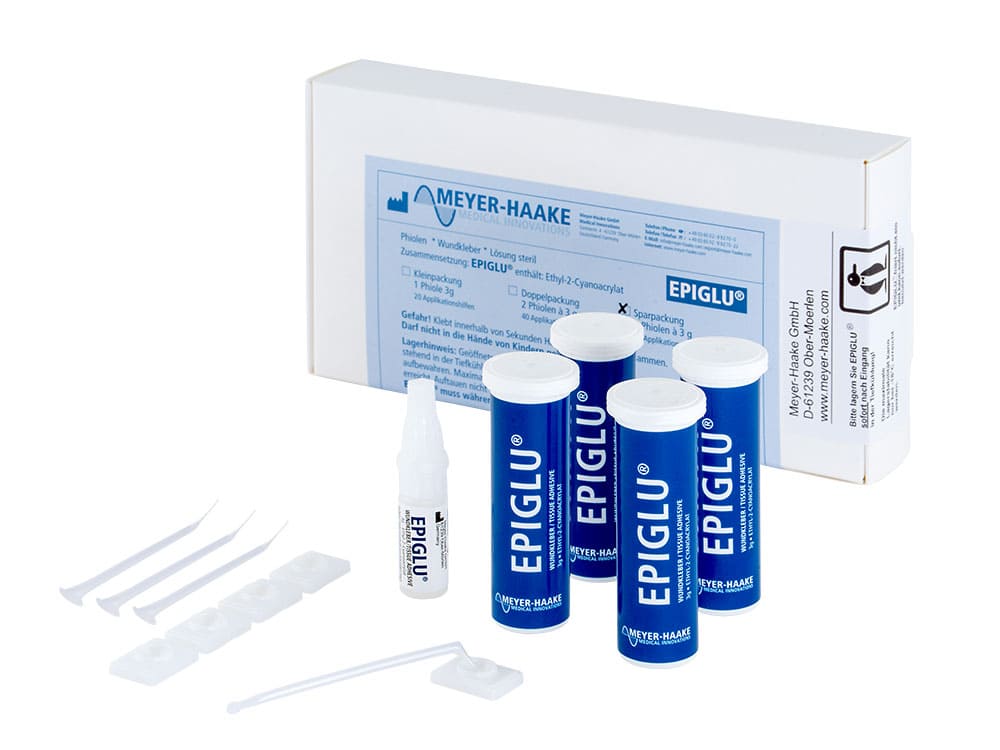
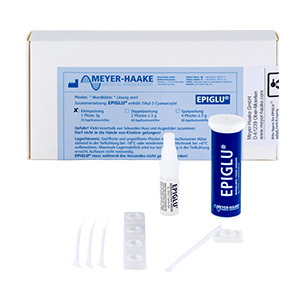
EPIGLU®
Adhesion instead of sutures or clamps
Modern wound treatment – innovative and economically
Request for a free sample and convince yourself.
Modern wound treatment – innovative and economically
The tissue adhesive EPIGLU® from Meyer-Haake more than fulfills the demands on modern and ideal wound treatment concerning barely visible scars and fast and problem-free healing. In comparison, laying stitches has necessarily disadvantages because removing of the sutures after the healing process is often associated with fear and time expenditure by the patient. Looking at the infection side, wound care is facing increasing difficult requirements. EPIGLU® secures the wound infection-secure.
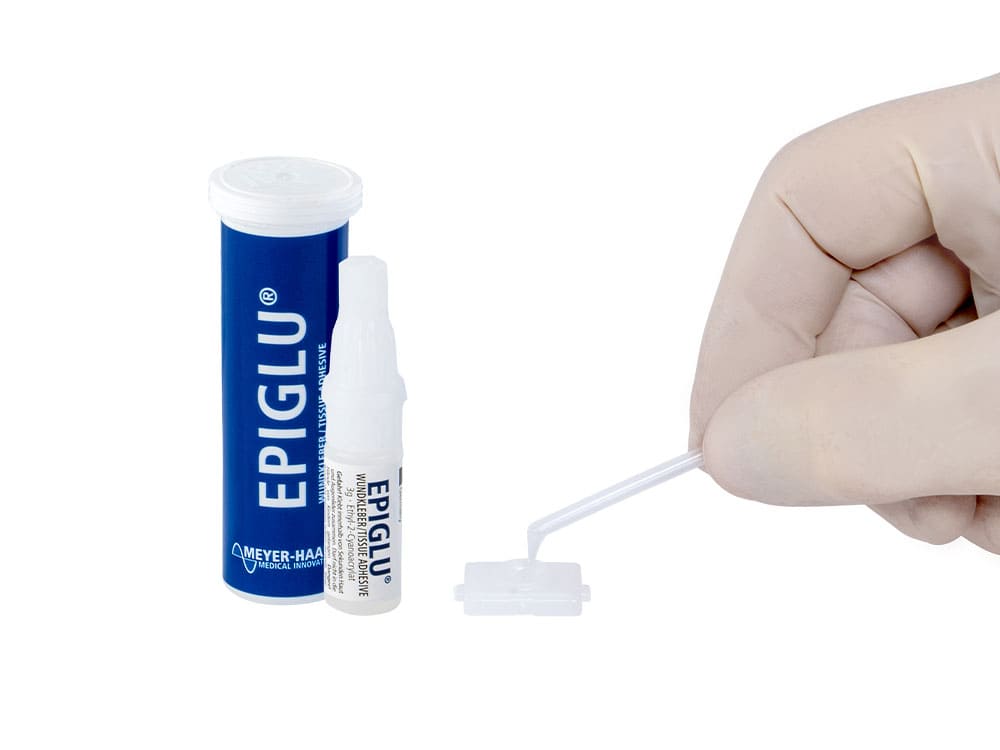
The Meyer-Haake solution for optimal wound treatment - the tissue adhesive EPIGLU®.
The ethyl-2-cyanoacrylate adhesive EPIGLU® with many advantages for physicians' offices, operation room, emergency room and care sector.
EPIGLU® is characterized by:
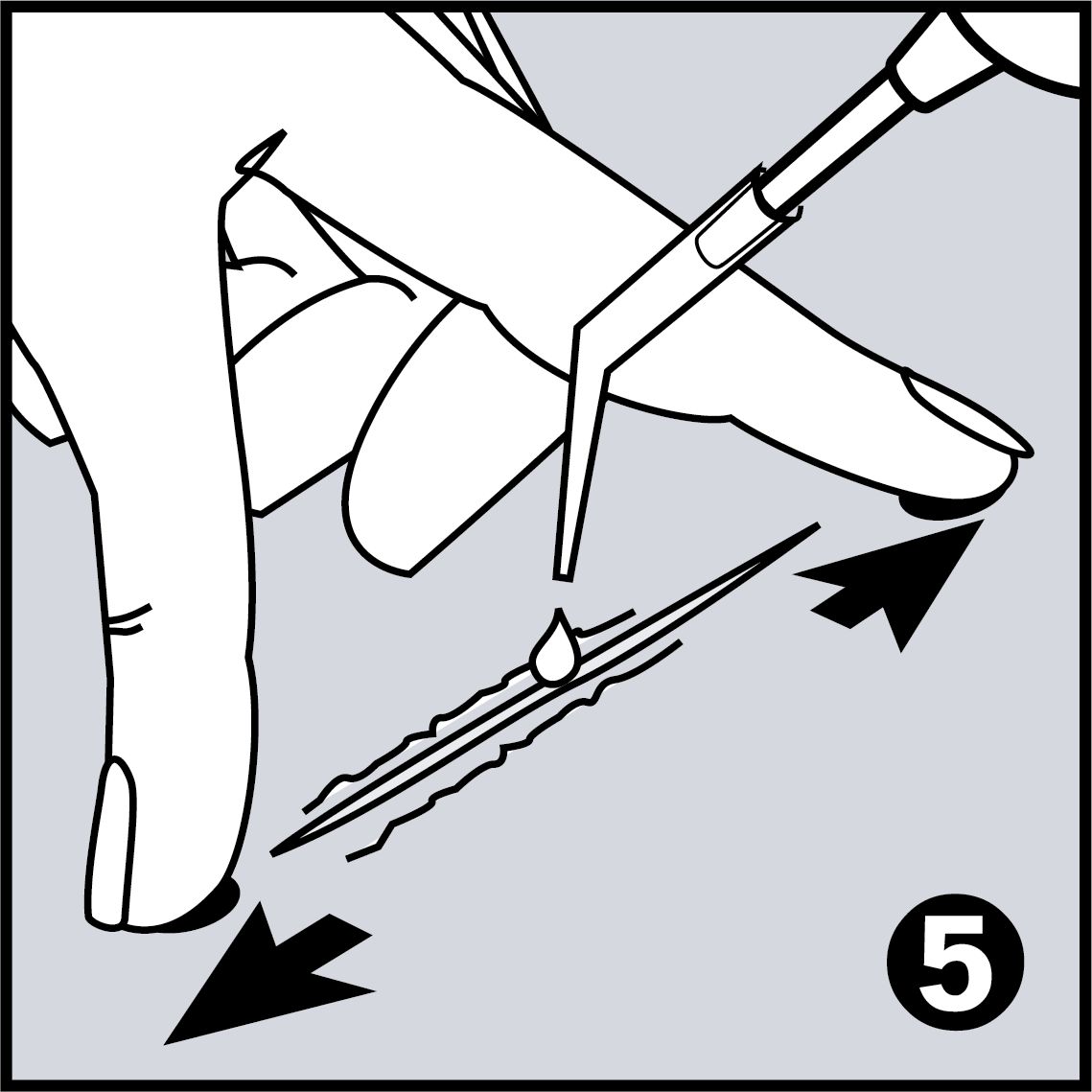
- Adheres wounds of every length and even wounds that are under tension
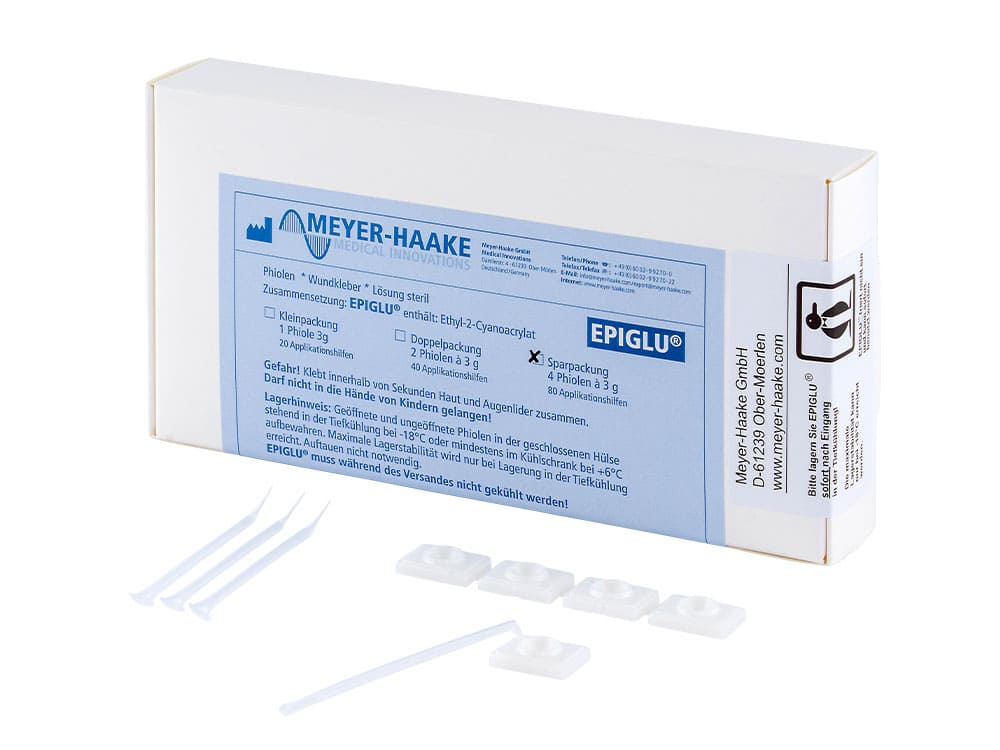
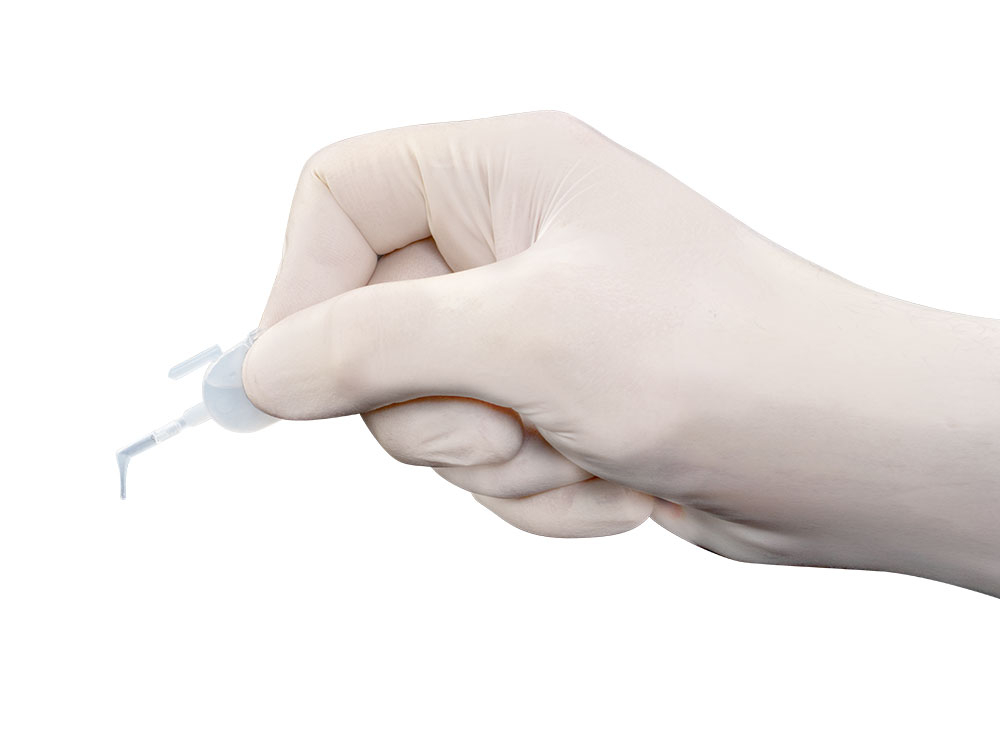
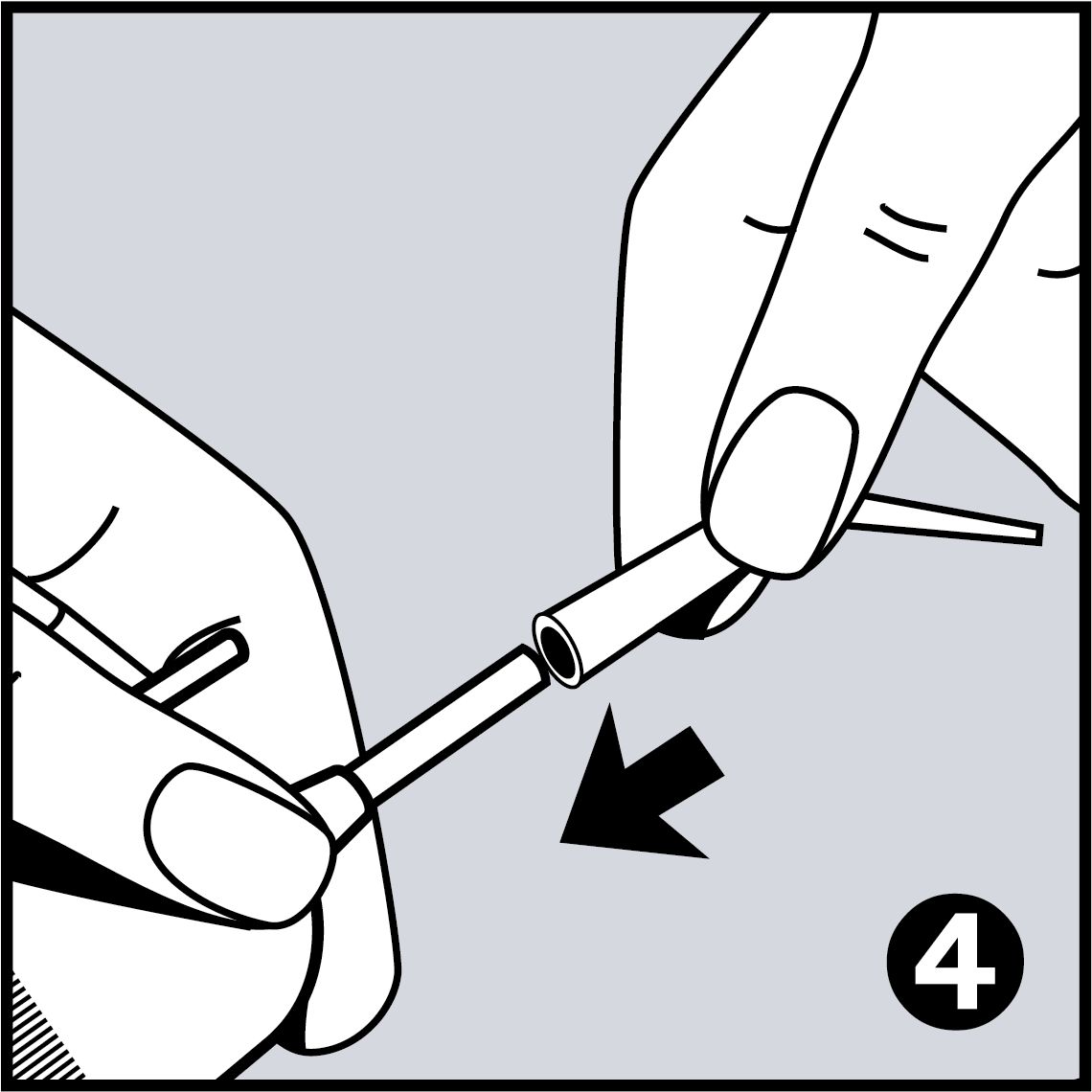
- Easy application with disposable pipettes
- Fast polymerization
- Long shelf life
- Favorable price / performance ratio
- User-friendly application sets
- Adheres even to difficult areas
- Protects the wound from infections
- Hardly any keloid formation
- Economic
- No allergic reactions known
- No burning sensation after application
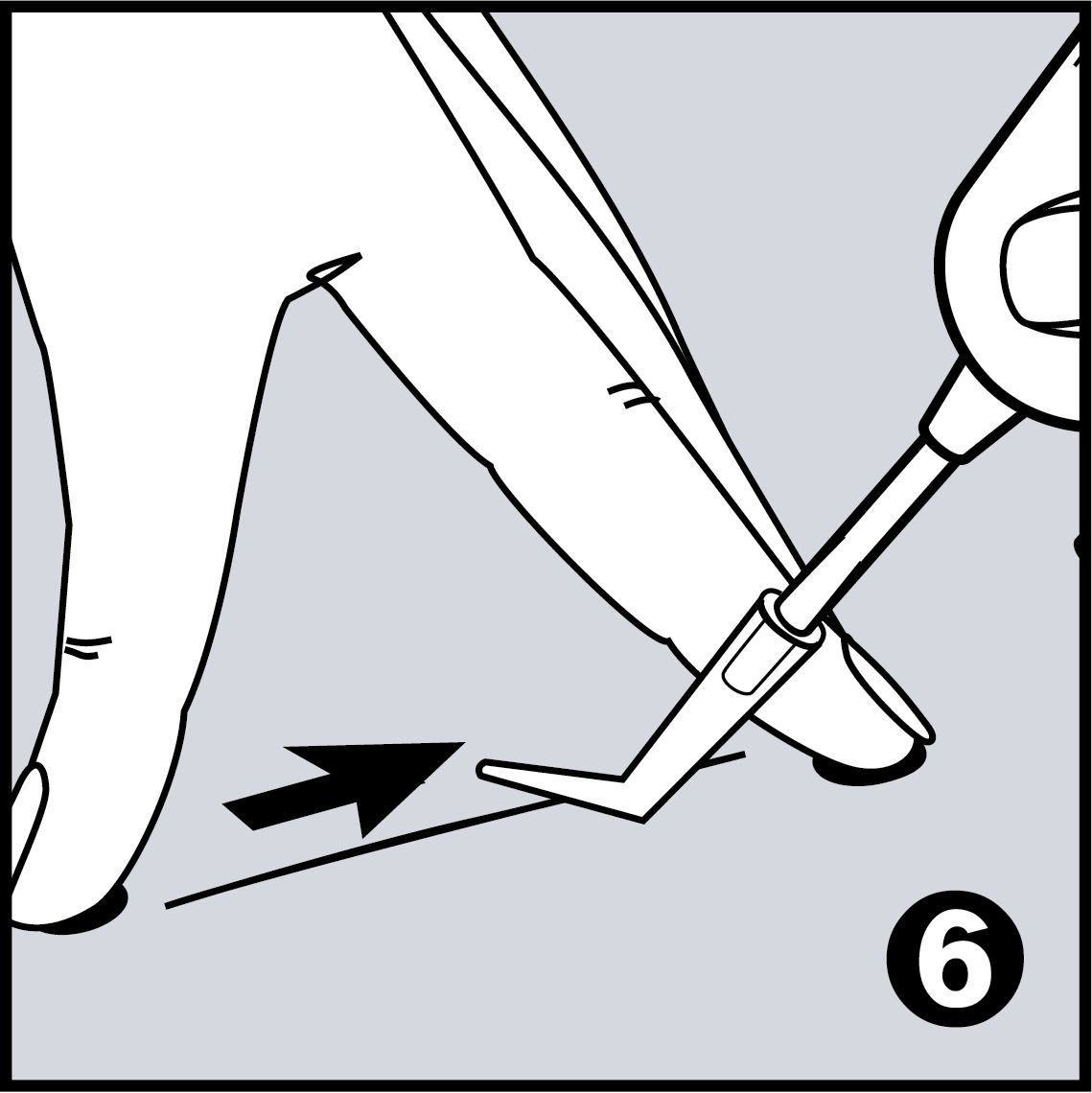
- No removal of the stitches – the tissue adhesive detaches after 8 to 10 days
- No follow-up treatment necessary
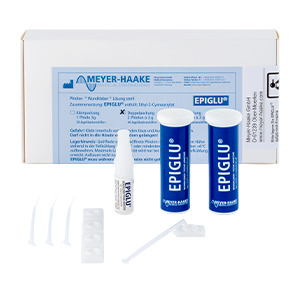
| EPIGLU® Wundkleber |
EPIGLU1P |
EPIGLU2P |
EPIGLU4P |
EPIGLUOS |
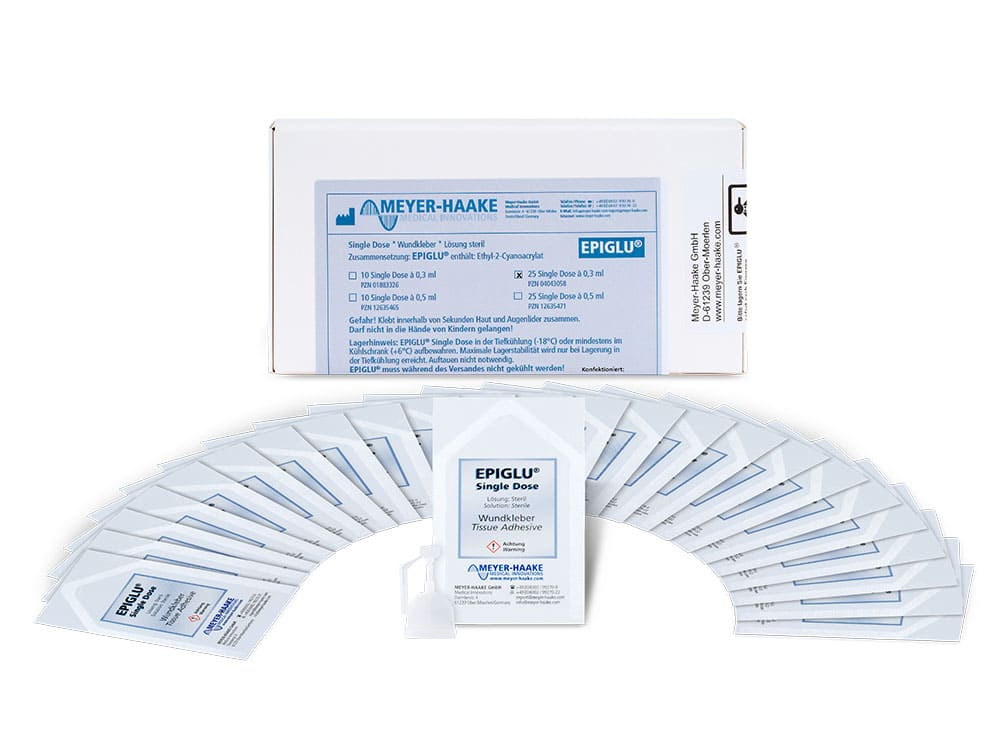
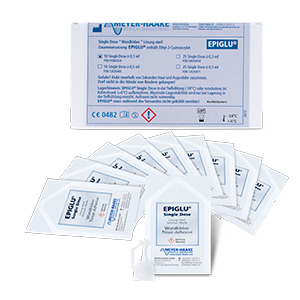
EPISDP10 |
EPISDP25 |
EPISP10F | EPISD25F |
|---|---|---|---|---|---|---|---|---|
|
|
 |
 |
 |
 |
 |
 |
 |
 |
| Content | 1 x 3g | 2 x 3g | 4 x 3g | 1 x 3g | 10 x 0,3ml | 25 x 0,3ml | 10 x 0,5ml | 25 x 0,5ml |
| Application sets | 20 nonsterile | 40 nonsterile | 80 nonsterile | 20 sterile | ||||
| Number of treatments | 20 | 40 | 80 | 20 | 10 x 1 | 25 x 1 | 10 x 1 | 25 x 1 |
| Self-sterilizing | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Adhesion lenght of application |
10cm | 10cm | 10cm | 10cm | 10-15cm | 10-15cm | 17-23cm | 17-23cm |
| Shelf life (-18°C) | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre |
| Polymerization | Sekunden | Sekunden | Sekunden | Sekunden | Sekunden | Sekunden | Sekunden | Sekunden |
| Use on mucosa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Antifungal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Antibacterial | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tear- and water resistant | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Approval for food animal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Suture protection | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Classification of medical device | IIb | IIb | IIb | IIb | IIb | IIb | IIb | IIb |
The ethyl-2-cyanoacrylate adhesive with many advantages for the dental practice and surgeries.
EPIGLU® is characterized by:
- Adheres also to the oral mucosa
- No disturbing threads
- Adheres wounds of every length – even under tension
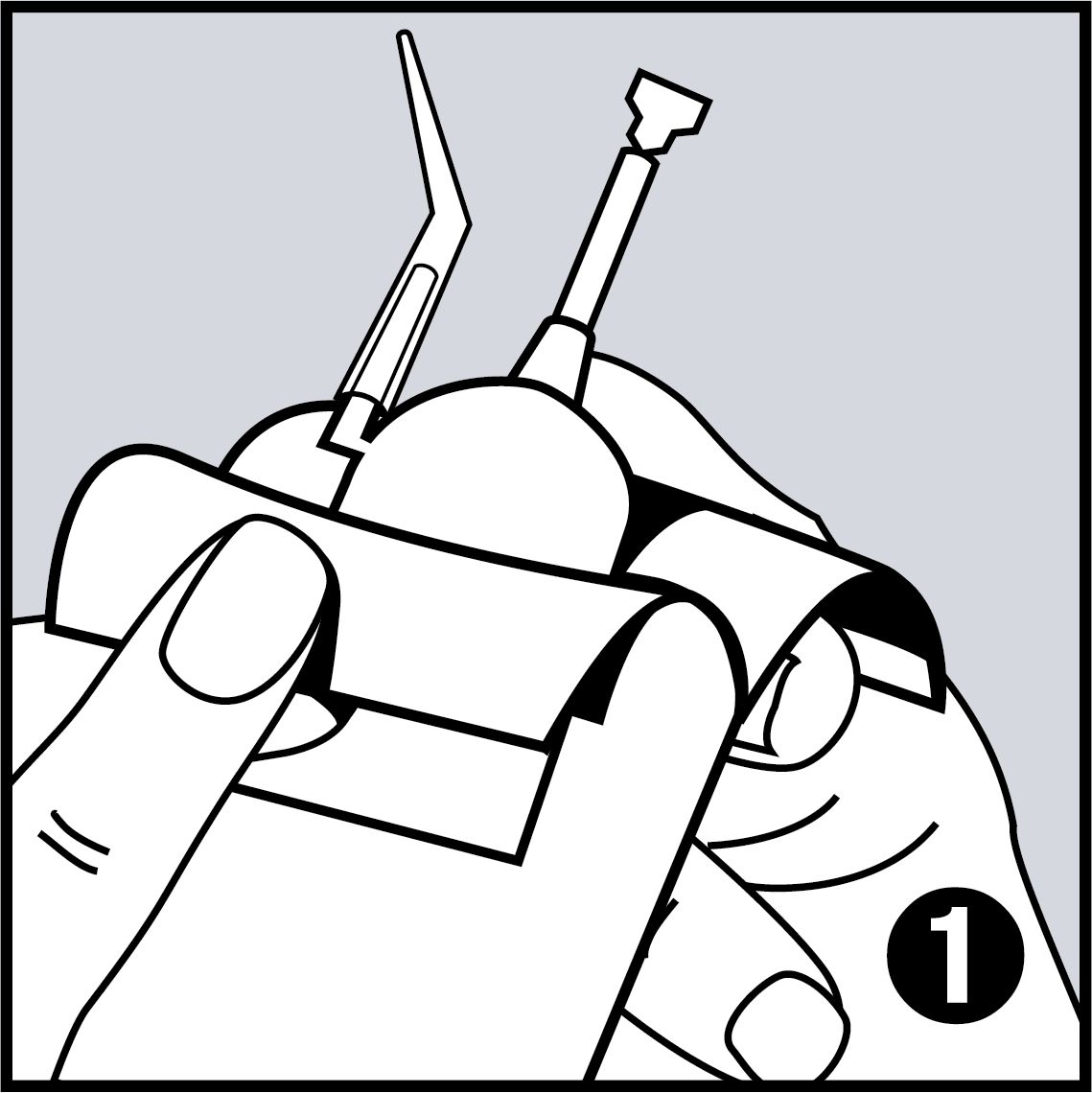
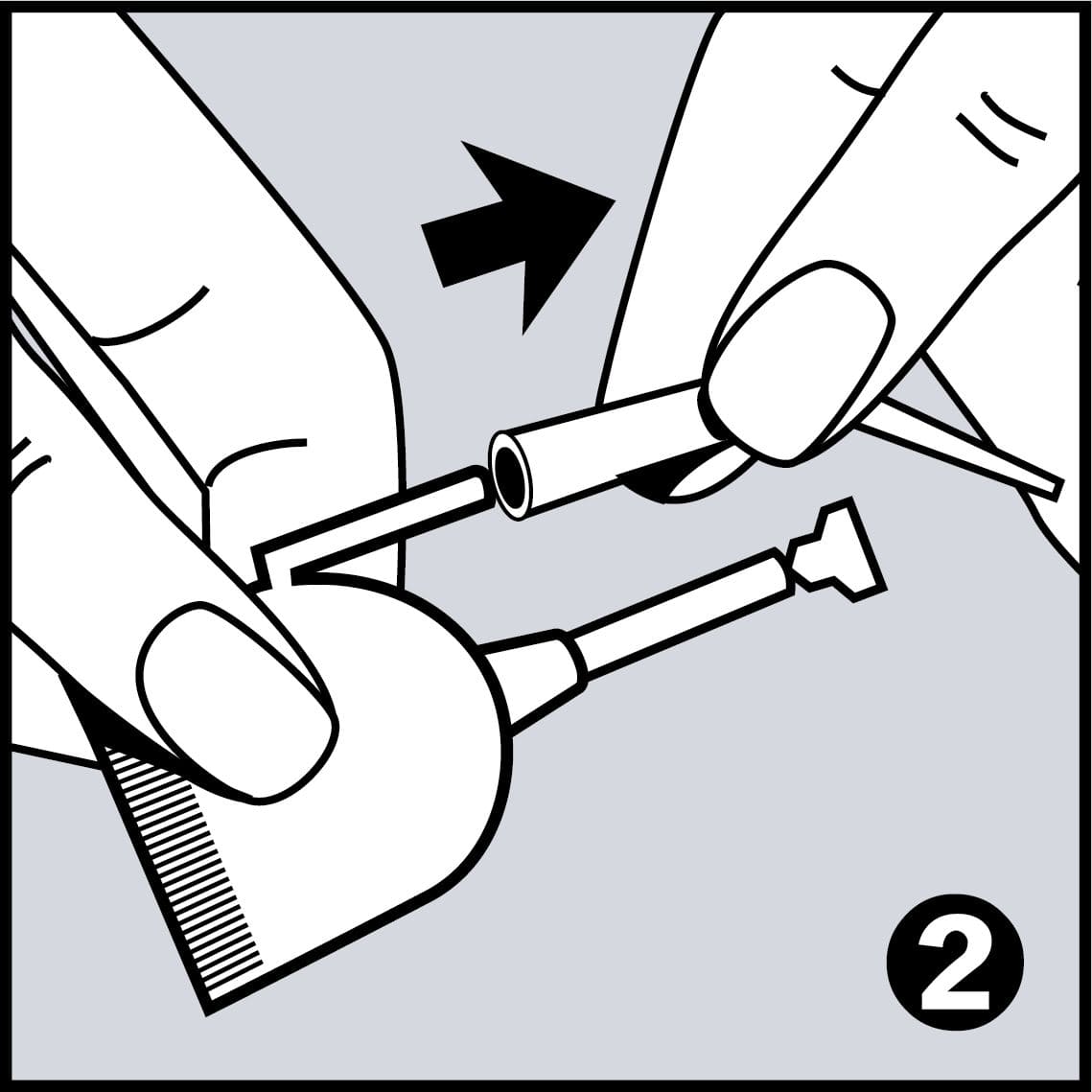
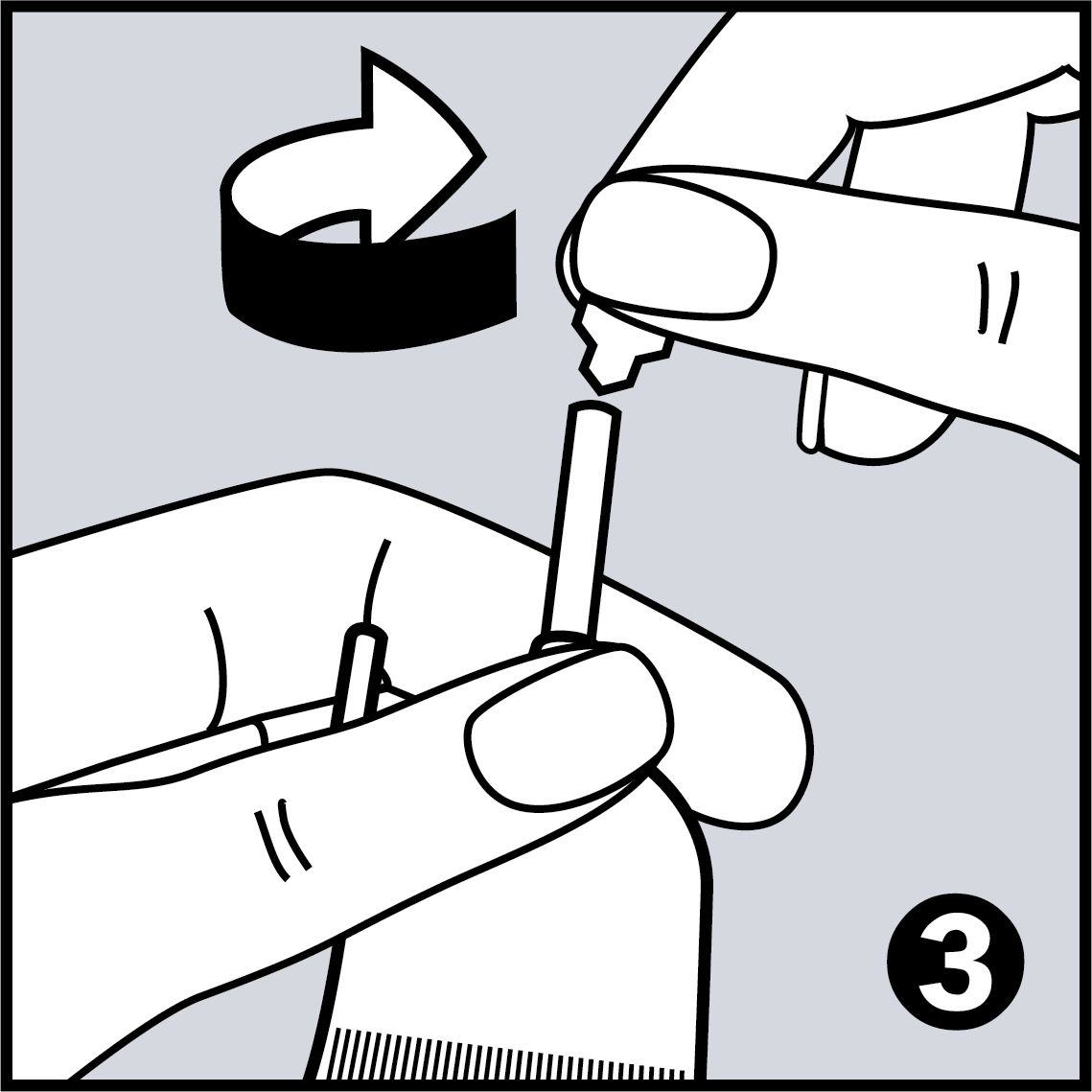
- Easy application with disposable pipettes, which are shaped like dental instruments, so every area in the mouth can be reached easily
- No tearing apart of thin mucosa membrane flaps when the needle is inserted
- Fast polymerization
- Long shelf life
- Favorable price / performance ratio
- User-friendly application sets
- Protects the wound from infections
- Economic
- No allergic reactions known
- Fast wound healing, even with aphthae
- No removal of the stitches – the tissue adhesive detaches after 8 to 10 days
- Different packaging sizes for every medical requirement
Very Easy to Use
 |
 |
 |
 |
 |
 |
| EPIGLU® Wundkleber |
EPIGLU1P |
EPIGLU2P |
EPIGLU4P |
EPIGLUOS |
EPISDP10 |
EPISDP25 |
EPISP10F | EPISD25F |
|---|---|---|---|---|---|---|---|---|
|
|
 |
 |
 |
 |
 |
 |
 |
 |
| Content | 1 x 3g | 2 x 3g | 4 x 3g | 1 x 3g | 10 x 0,3ml | 25 x 0,3ml | 10 x 0,5ml | 25 x 0,5ml |
| Application sets | 20 nonsterile | 40 nonsterile | 80 nonsterile | 20 sterile | ||||
| Number of treatments | 20 | 40 | 80 | 20 | 10 x 1 | 25 x 1 | 10 x 1 | 25 x 1 |
| Self-sterilizing | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Adhesion length per application | 10cm | 10cm | 10cm | 10cm | 10-15cm | 10-15cm | 17-23cm | 17-23cm |
| Shelf life (-18°C) | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre | 2 Jahre |
| Polymerization | Sekunden | Sekunden | Sekunden | Sekunden | Sekunden | Sekunden | Sekunden | Sekunden |
| Use on mucosa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Antifungal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Antibacterial | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tear and water resistant | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Approval for food animal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Suture protection | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Classification of medical device | IIb | IIb | IIb | IIb | IIb | IIb | IIb | IIb |